Abstract
BACKGROUND:
Eltrombopag is an oral, small-molecule, non-peptide thrombopoietin receptor agonist (TPO-RA) that increases hematopoiesis by inducing proliferation and differentiation of early bone marrow progenitor cells leading to increased platelet production (Erickson-Miller et al., 2010, Sun et al., 2012). Eltrombopag has demonstrated efficacy in adult and pediatric patients with immune thrombocytopenia (ITP) and has a well-established safety profile. It is approved in Europe for the treatment of thrombocytopenia, from 6 months following diagnosis, in patients with primary ITP who are refractory to other treatments (e.g. corticosteroids, immunoglobulins).
The efficacy of eltrombopag for achieving hemostatic platelet counts (≥ 50 × 10 9/L) in previously treated adult ITP patients with more than 6 months' disease duration is around 80 % (Wong et al., 2017). However, there is insufficient data on safety and efficacy of TPO-RAs in newly diagnosed ITP patients.
AIM:
The aim of this trial (NCT04346654; CETB115JDE01) is to compare the ability of eltrombopag in combination with a short course of high-dose dexamethasone to induce a sustained response off treatment in comparison to dexamethasone monotherapy in newly diagnosed primary ITP patients.
METHODS:
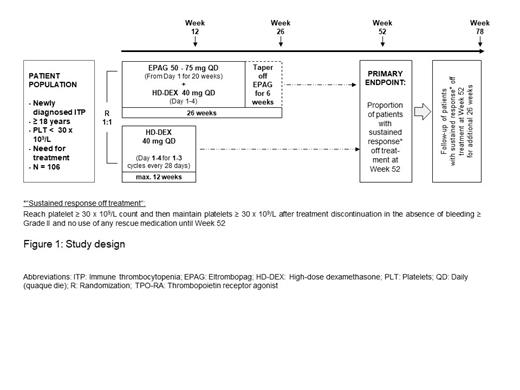
This is a Phase II, multicenter, randomized (1:1), open-label study (see Figure 1).
Arm A: Eltrombopag + short course of high-dose dexamethasone
Arm B: 1-3 cycles of high-dose dexamethasone
Adult patients with newly diagnosed primary ITP who have platelet counts < 30 × 10 9/L and require treatment will be screened, and if eligible, will be randomized to either Arm A or Arm B.
The study will be conducted in the following periods:
Treatment / Tapering Period:
Arm A: Patients will receive eltrombopag in combination with a short course of high-dose dexamethasone beginning at day 1. The dose of dexamethasone will be 40 mg QD (daily; quaque die) for 4 consecutive days and limited to 1 cycle. The starting dose of eltrombopag will be 50 mg QD in order to achieve the target platelet count of ≥ 50 × 10 9/L.
Patients who reach platelet counts ≥ 30 × 10 9/L and maintain counts ≥ 30 × 10 9/L during the tapering phase (week 20 - week 26) will be eligible for treatment discontinuation starting from week 26.
During the tapering phase, eltrombopag will be decreased by 25 mg every 2 weeks to a minimum dose of 25 mg every other day for all patients.
Arm B: Treatment in the control arm consists of 1-3 cycles of high-dose dexamethasone administered orally at a dose of 40 mg QD for 4 consecutive days at 2-4 week intervals. Patients will be treated up to 12 weeks during the treatment period with dexamethasone. If the platelet counts are > 150 × 10 9/L no further course of dexamethasone will be given. Patients who reach platelet counts ≥ 30 × 10 9/L and maintain counts ≥ 30 × 10 9/L after 1-3 cycles of dexamethasone treatment will be eligible for treatment discontinuation.
Observation period: After completion of the treatment period, patients will be observed for sustained response off treatment defined as:
maintain platelet counts ≥ 30 × 10 9/L after treatment discontinuation and
no bleeding events ≥ Grade II and
without the use of any rescue therapy until week 52 and week 78 respectively, after study start
The study is designed to include 106 adult patients with newly diagnosed primary ITP at 30 sites in Germany.
Patients meeting any of the following criteria are not eligible for inclusion in this study:
Previous history of treatment for ITP except 3 days of ITP rescue medication within 7 days before study randomization
Patients with diagnosis of secondary thrombocytopenia
Patients who have life threatening bleeding complications per physician´s discretion
Patients with a history of thromboembolic events or known risk factors for thromboembolism
The primary objective is the rate of sustained responses off treatment at 52 weeks. Key secondary objectives include the duration of sustained response off treatment, the rate of sustained response off treatment at 78 weeks as well as patient-oriented outcomes for health-related quality of life.
Currently, the study is recruiting patients, expected to be completed by 2022.
CONCLUSION:
This trial will evaluate the potential of eltrombopag in combination with steroids to increase the rate of sustained response off treatment in comparison to steroids alone in patients with previously untreated primary ITP.
Binder: Deutsche Krebsgellschaft, Medconcept GmbH, event Lab. GmbH: Honoraria, Other: Speaker Activity; Amgen GmbH, Janssen-Cilage GmbH, DGHO, Art tempi, Tumorzentrum Anhalt MD, Uniklinikum Hamburg, Sanofi Aventis: Honoraria, Other: Speaker Activity. Matzdorff: Amgen: Consultancy, Honoraria; Argenx: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Grifols: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Current holder of individual stocks in a privately-held company; UCB: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Meyer: Novartis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Grifols: Consultancy, Honoraria; SOBI: Consultancy, Honoraria. Tesanovic: Novartis Pharma GmbH: Current Employment. Sauer: Novartis Pharma GmbH: Current Employment.
Eltrombopag is approved in Europe for the treatment of thrombocytopenia, from 6 months following diagnosis, in patients with primary ITP who are refractory to other treatments (e.g. corticosteroids, immunoglobulins). In this study Eltrombopag will be administered as first-line therapy in ITP.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal